Stability Study Expertise at Valentia Analytical
What are Stability Studies?
Stability studies are scientific investigations that determine the shelf life and expiration date of a product by assessing how its quality, safety, and efficacy change over time under various environmental conditions. These studies help ensure that the product remains effective and safe for use throughout its intended storage period. The primary types of stability studies include:
- Long-Term Studies: Conducted under normal storage conditions to monitor the product's stability over its intended shelf life.
- Accelerated Studies: Performed under elevated stress conditions (higher temperature and humidity) to predict shelf life in a shorter period.
- Stress Testing: Subjects the product to extreme conditions to identify potential degradation pathways and establish product robustness.
Additionally, Valentia Analytical customizes studies such as in the application of Accelerated Predictive Stability (APS) studies to provide earlier indications of a product's stability through modeling and simulation of real-world conditions.
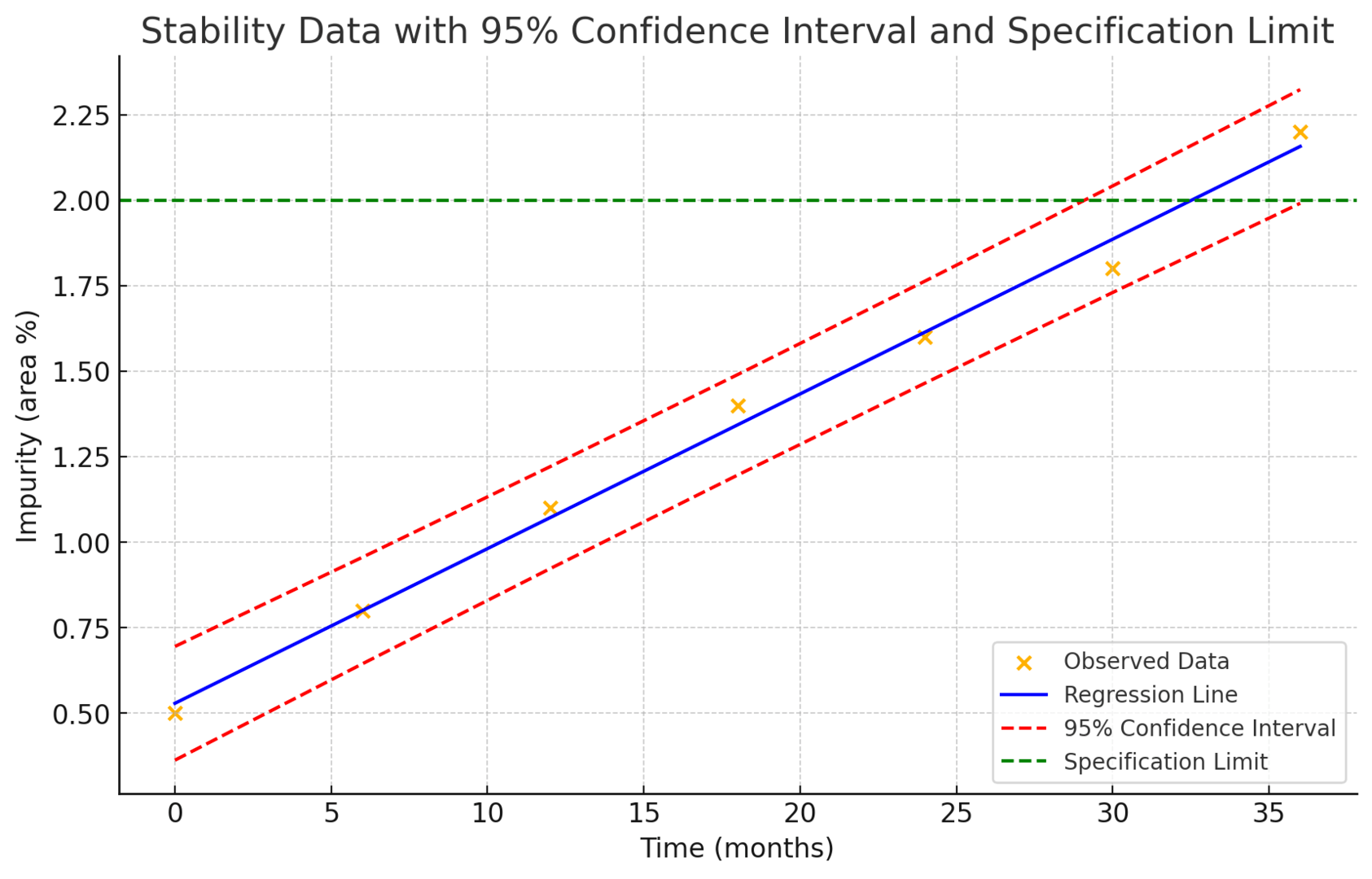
Our Expertise
Our expertise lies in the management of stability programs and the analytical analysis required to ensure product integrity. We utilize state-of-the-art equipment and technologies, including ultra-high-performance liquid chromatography (UHPLC), capillary electrophoresis (CE), cell-based bioassays, ELISAs, and more. Our team provides critical reviews of the data to ensure integrity throughout the process, integrating all data to present a clear and comprehensive understanding of the product's stability profile.

Services Offered
Valentia Analytical offers comprehensive stability study services, including:
- Stability Program Management: Managed through a robust Quality Management System (QMS) to ensure compliance and reliability.
- Regulatory Support: Assistance with the preparation of stability study reports and documentation for regulatory submissions.
- On-Site Storage and Testing: Controlled storage environments and comprehensive testing capabilities to simulate real-world conditions and assess product stability accurately.
By emphasizing these key areas, Valentia Analytical demonstrates its commitment to providing high-quality stability studies that meet industry standards and support the development and commercialization of safe, effective products.