TESTING
Samples can be tested for content, impurities, and identity. Valentia Analytical employs its state of the art chromatographic ultra-performance liquid chromatographic systems to separate, detect, and quantify analytes.
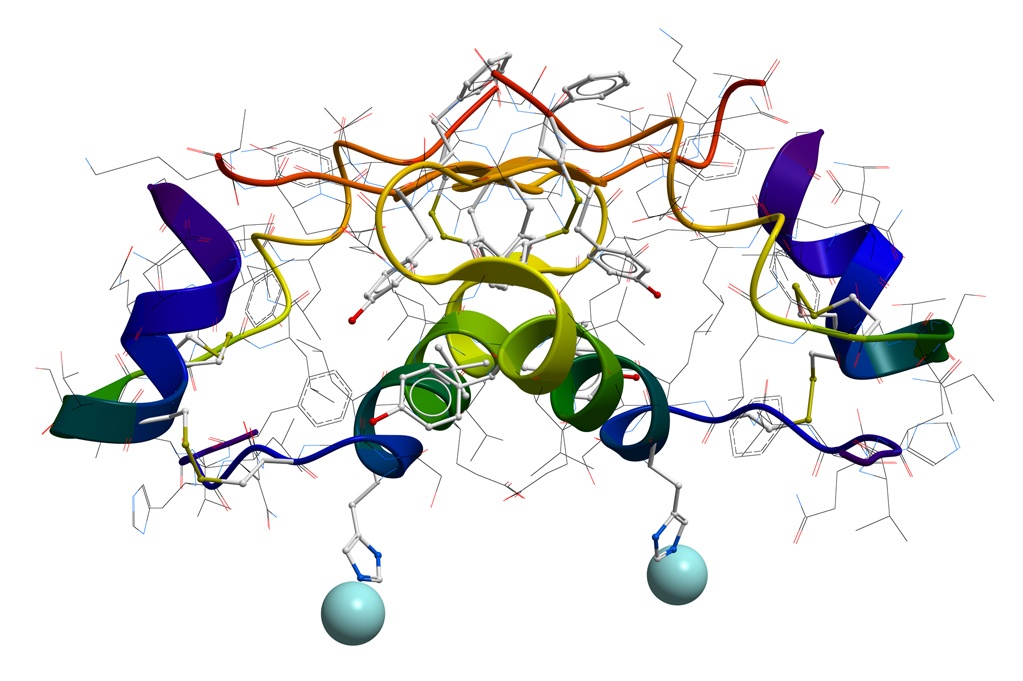
PROTEINS
Attributes tested and characterized in proteins include purity, potency, impurities, degradation, charged-heterogeneity. IgG monoclonal antibodies are a class of proteins typically analyzed for these characteristics. Examples of the testing we would conduct are size exclusion measuring levels aggregation or fragments; ion exchange measuring charged heterogeneity, or purity by reversed-phase chromatography. We have studied impact of irradiation on protein structure. Affinity chromatogram can be developed into in-process methods used to measure IgG titer concentration.
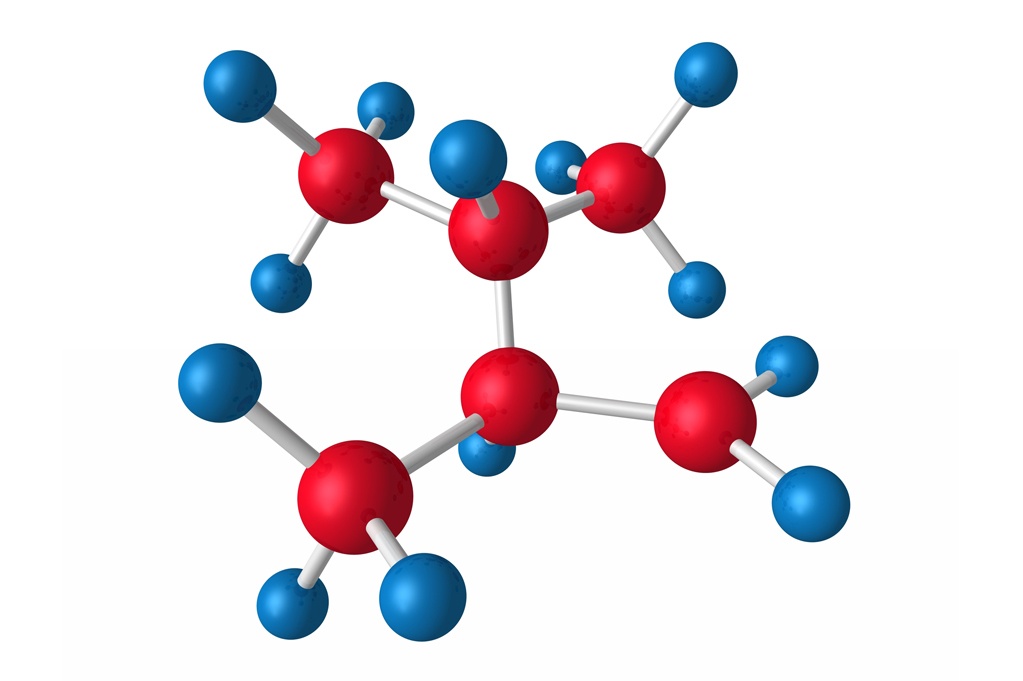
SMALL MOLECULE
Reversed-phase is often used to characterize small molecules. Methods are developed for raw materials, in-process, assay, impurities, and degradation products. Stressed studies would be performed in accordance with ICH Q1 to develop stability-indicating assays and determine critical quality attributes. The application of UPLC is capable of speeding up analysis 6-fold and providing high sensitivity for low-level analytes.

DNA
Plasmid DNA should be assessed for topoisomeric purity. Special columns that take advantage of charge density differences between such forms are employed toward the separation and characterization of these supercoiled, open chain, and linear forms. Data are coupled with orthoganol methods including gel electrophoresis for the analysis of compounds.
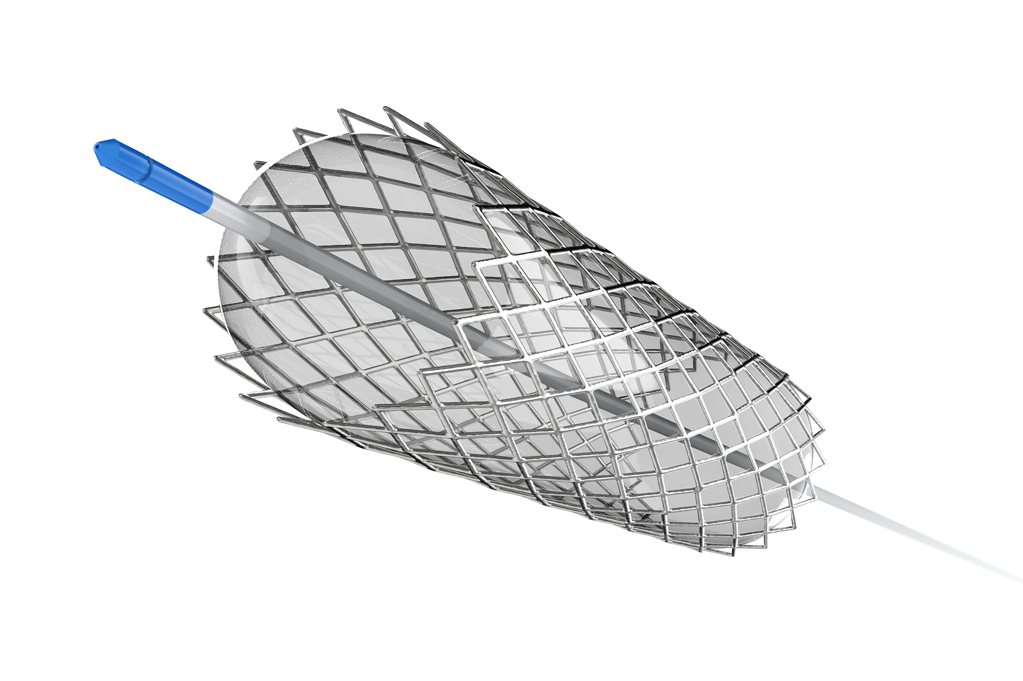
COMBINATIONAL MEDICAL DEVICE
Combination medical devices with drug and/or protein have become highly successful products. Typical quality attributes include drug load, drug release, impurities, and degradation products. Furthermore, impact of terminal sterilization in additional to stability must be characterized.

RAW MATERIALS
Incoming raw materials require identification by either a specific method or various non-specific ones. UPLC/HPLC is common to such use. Additional non-chromatographic methods can be employed perform such testing.